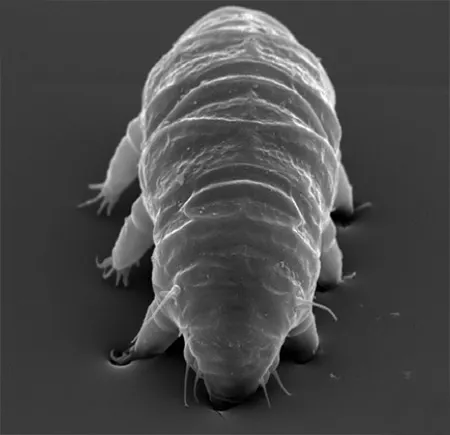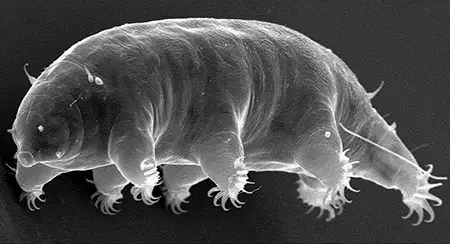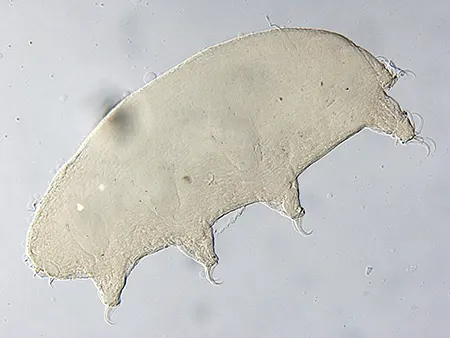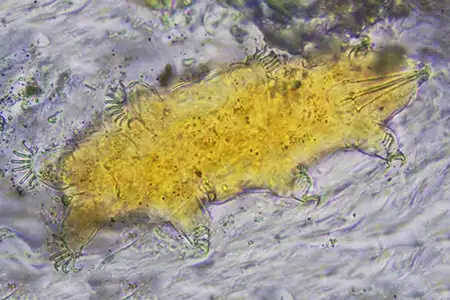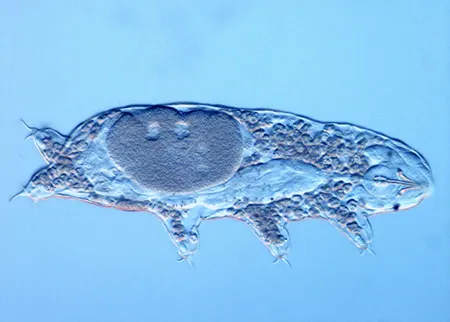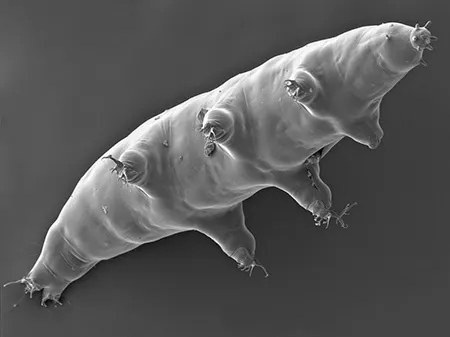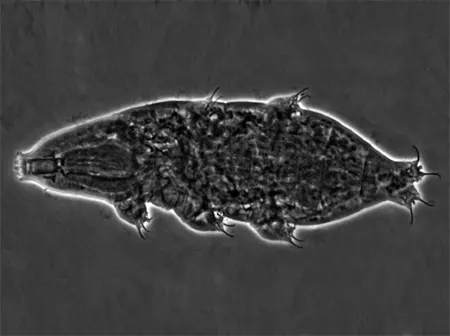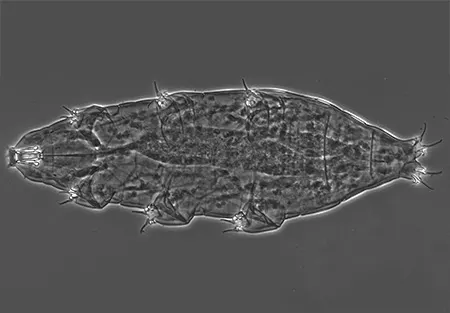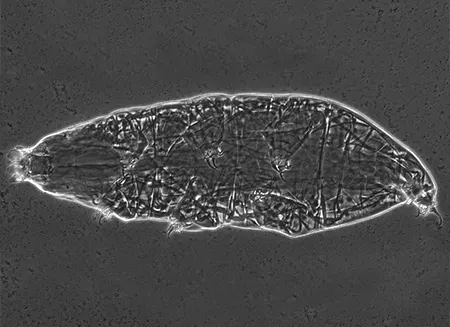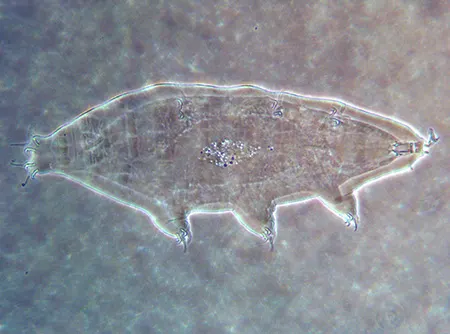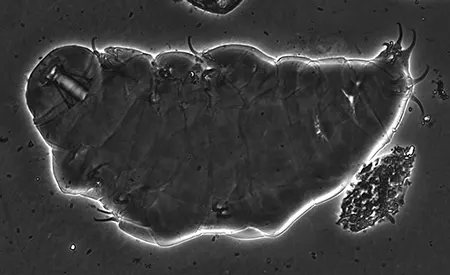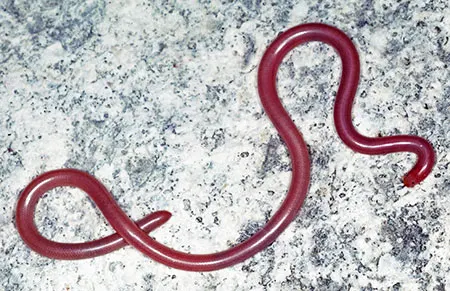
(Leptotyphlopidae)
Thread Snakes
Стрункі сліпуни
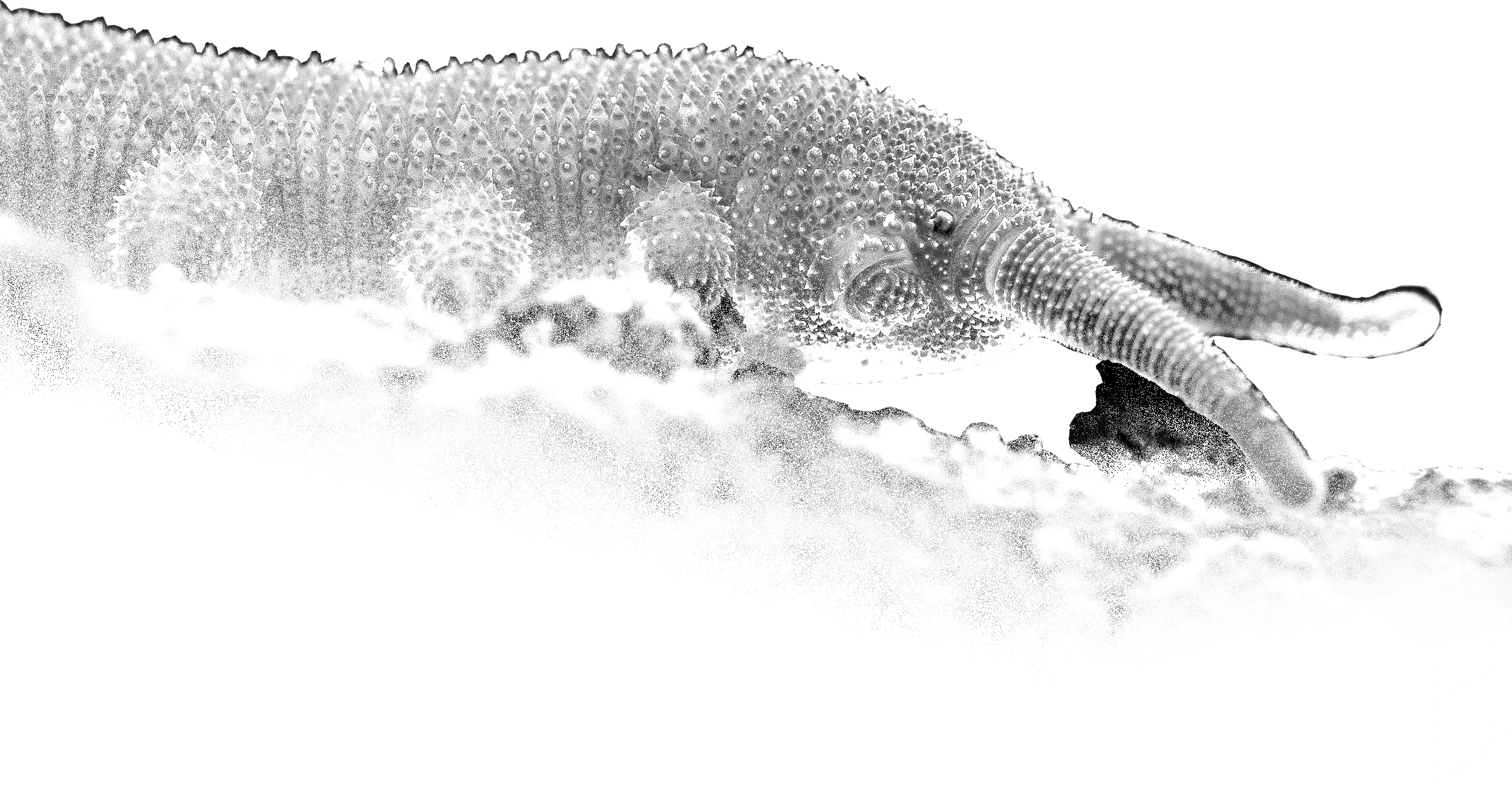
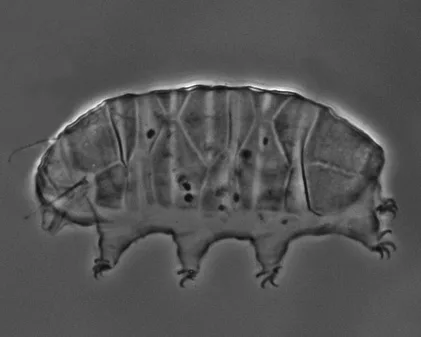
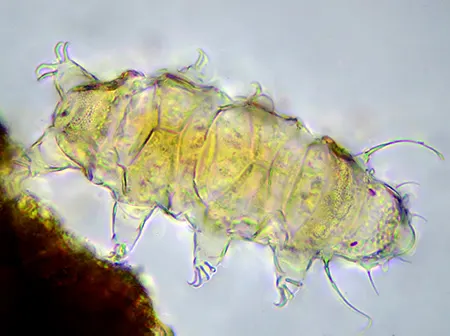
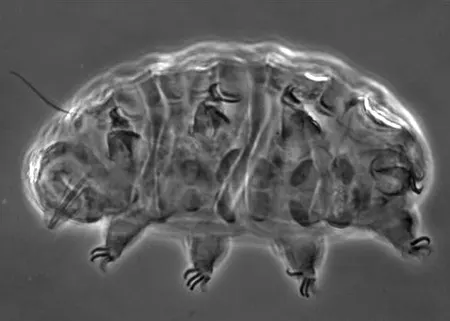
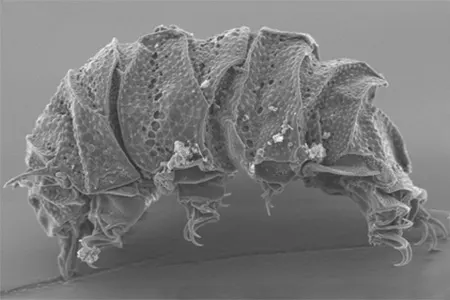
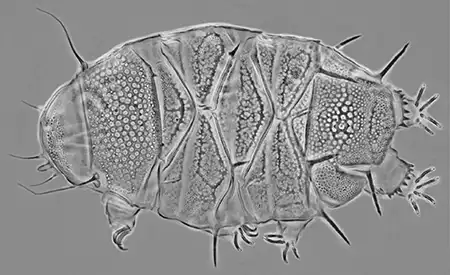
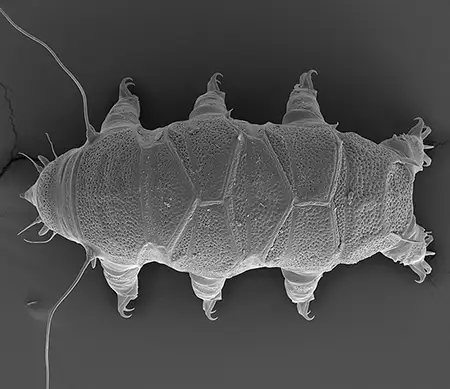
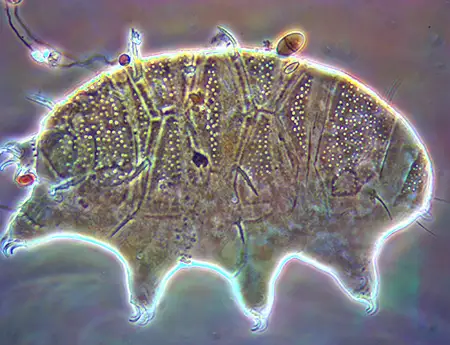
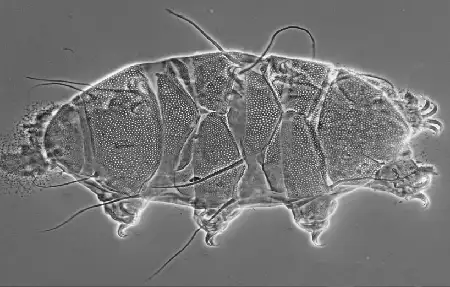
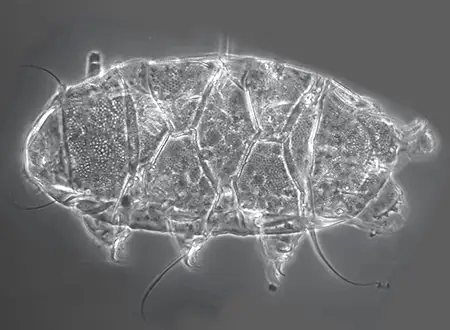
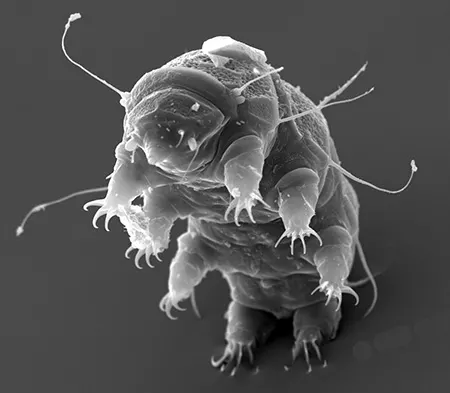
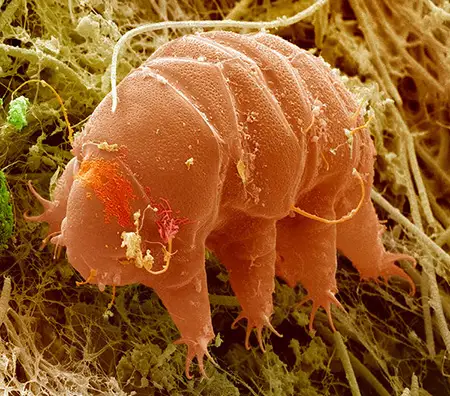
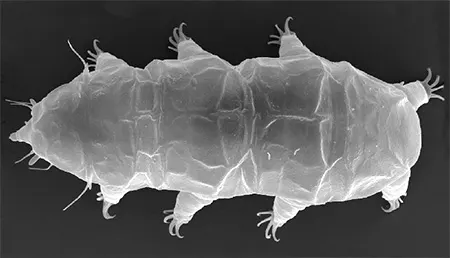
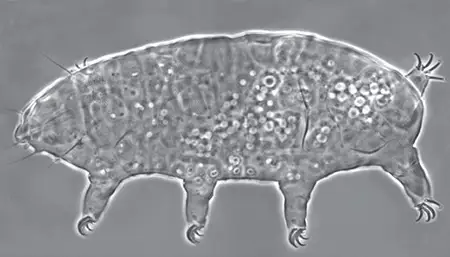
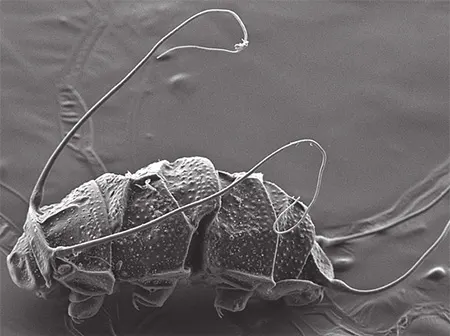
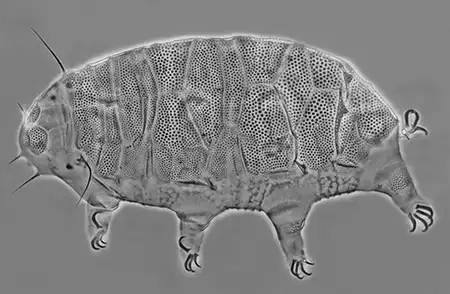
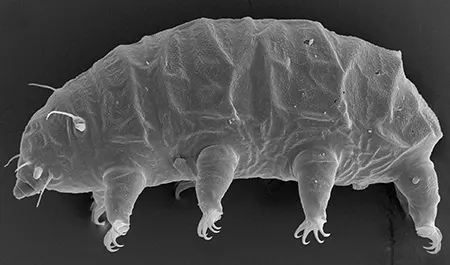
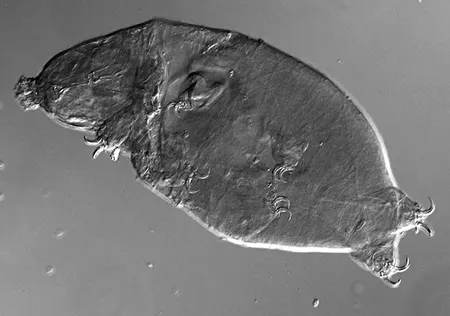
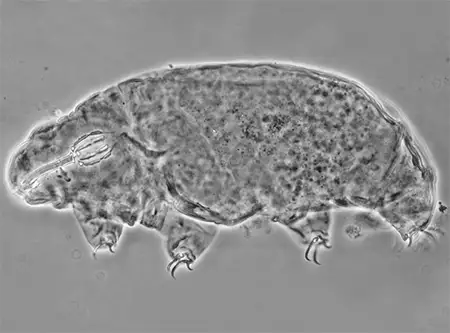
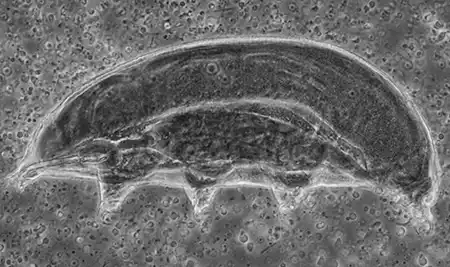
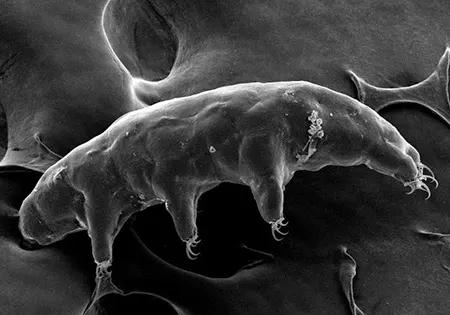
The Leptotyphlopidae (commonly called slender blind snakes or thread snakes) are a family of small snakes found in North America, South America, Africa and Asia. All are fossorial and adapted to burrowing, feeding on ants and termites. Two subfamilies are recognized: Leptotyphlopinae and Epictinae.
Relatively small snakes, leptotyphlopids rarely exceed 30 cm in length; only Trilepida macrolepis and Namibiana occidentalis grow larger. The cranium and upper jaws are immobile and no teeth are in the upper jaw. The lower jaw consists of a much elongated quadrate bone, a tiny compound bone, and a relatively larger dentary bone. The body is cylindrical with a blunt head and a short tail. The scales are highly polished. The pheromones they produce protect them from attack by termites. Among these snakes is what is believed to be the world’s smallest: Tetracheilostoma carlae.
Leptotyphlopids are found in Africa, western Asia from Turkey to eastern India, on Socotra Island, and from the southwestern United States south through Mexico and Central America to South America, though not in the high Andes. In Pacific South America, they occur as far south as southern coastal Peru, and on the Atlantic side as far as Uruguay and Argentina. In the Caribbean, they are found on the Bahamas, Hispaniola, and the Lesser Antilles.
Leptotyphlopids occur in a wide variety of habitats from arid areas to rainforest, and are known to occur near ant and termite nests.
The diets of leptotyphlopids consist mostly of termite or ant larvae, pupae, and adults. Most species suck out the contents of insect bodies and discard the exoskeleton.
Snakes in the family Leptotyphlopidae are oviparous. Females lay 2 to 6 eggs.

(Epictia albifrons)
Wagler's Blind Snake
Стрункий сліпун Ваглера
It is endemic to the Brazilian state of Pará. It inhabits humid lowland tropical forests and is also found on plantations, in gardens, and near human settlements at elevations of up to 500 m.

(Epictia albipuncta)
Latin American Blind Snake
Стрункий сліпун латиноамериканський
It is found in Argentina, Bolivia, south-western Brazil, Paraguay, Uruguay, and possibly southern Peru. It is found grassland, shrubland, savanna, and forest.

(Epictia alfredschmidti)
It is known from the type locality in Huarmey Province, western Peru. It inhabits Andean mountain steppes at elevations of 2,940–3,090 m.

(Epictia amazonica)
South American Blind Snake
Стрункий сліпун південноамериканський
It is found in Colombia, French Guiana, Guyana, and southeastern Venezuela (Amazonas, Bolívar). It inhabits humid tropical forests.

(Epictia antoniogarciai)
García's Blind Snake
Стрункий сліпун Гарсії
It is known from a few specimens collected in the Cajamarca and Amazonas regions of northern Peru. It inhabits the dry forests of the Marañón Valley.

(Epictia ater)
Taylor's Blind Snake
Стрункий сліпун Тейлора
It is found in western and northern Honduras and along the Pacific coast of Nicaragua and Costa Rica. It inhabits dry tropical forests in the lowlands and humid tropical forests in the foothills at elevations of up to 1,340 m.

(Epictia australis)
Freiberg's Blind Snake
Стрункий сліпун Фрайберга
It is found in Argentina (Río Negro, Mendoza, La Pampa, and Córdoba), Bolivia (Beni), and Brazil (Rio Grande do Sul). It inhabits shrubland, grassland, and the savannas of the Monte, Espinal, and Chaco.

(Epictia bakewelli)
Bakewell’s Blind Snake
Стрункий сліпун Бейквелла
It is found in Mexico in coastal and foothill regions on Pacific slopes from Colima and Michoacan southward to the Isthmus of Tehuantepec.

(Epictia borapeliotes)
It is found in northeastern Brazil, in the states of Ceará, Rio Grande do Norte, Paraíba, Pernambuco, Alagoas, Sergipe, and Bahia. It inhabits dry caatinga scrubland, occurring in the agreste and in humid coastal areas.

(Epictia clinorostris)
It is known from two localities in central Brazil, located in south-eastern Mato Grosso and western Goiás. t inhabits gallery forests surrounded by cerrado savannas.

(Epictia columbi)
San Salvador Blind Snake
Стрункий сліпун сан-сальвадорський
It is endemic to San Salvador Island in The Bahamas, where it inhabits forest and shrubland.

(Epictia diaplocia)
Common Peru Blind Snake
Стрункий сліпун перуанський
It is found in north-eastern Peru. It inhabits humid lowland tropical forests at elevations of 100–200 m.

(Epictia fallax)
Chacachacare Thread Snake
It is known only from Chacachacare Island, Venezuela. It inhabits dry tropical forests and open areas.

(Epictia goudotii)
Southern Caribbean Threadsnake
Стрункий сліпун південно-карибський
It is found in the Magdalena River valley of Colombia, along the Caribbean coast of Colombia and Venezuela, on the adjacent islands of Margarita and Trinidad, and along the coast of the Gulf of Panama in Panama.

(Epictia magnamaculata)
Western Caribbean Threadsnake
Стрункий сліпун західно-карибський
It is found on the island of Cozumel off Mexico; on the Islas de la Bahía, Cayos Cochinos, and the Swan Islands off the coast of Honduras; and on the Colombian islands of San Andrés, Providencia, and Santa Catalina. It inhabits tropical forests and coconut groves.

(Epictia munoai)
Rio Grande Do Sul Blind Snake
Стрункий сліпун Муньоа
It is found in northern Argentina, extreme southern Brazil, and Uruguay, where it inhabits forest and grassland habitats.

(Epictia pauldwyeri)
It is found on the Pacific coast of central Panama. It inhabits humid lowland tropical forests at elevations of up to 250 m.

(Epictia phenops)
It is found in El Salvador, Guatemala, Belize, Honduras, and southern Mexico. It occurs in a wide range of habitats, from savanna to montane rainforest.

(Epictia resetari)
It is found in southern Tamaulipas, Veracruz, and north-eastern Hidalgo, Mexico. It inhabits evergreen and semi-deciduous tropical forests and also occurs in savannas at elevations of up to 900 m.

(Epictia rufidorsa)
Rose Blind Snake
It is known from several localities along the coast of Peru in the Lima and La Libertad regions. It inhabits the coastal Sechura Desert.

(Epictia schneideri)
It is found in Mexico in the Sierra Madre del Sur Mountains in the states of Guerrero and Oaxaca. It inhabits pine–oak, deciduous, and semi-deciduous forests at elevations of 825–1,675 m.

(Epictia septemlineata)
It is known from the type locality in Celendín Province, Cajamarca Region, northern Peru, at an elevation of 2,053 m.

(Epictia striatula)
It is found on the eastern slopes of the Andes in extreme southern Bolivia (Tarija) and northern Argentina (Salta). It inhabits the humid tropical forests of the Yungas.

(Epictia subcrotilla)
Klauber's Blind Snake
Стрункий сліпун Клаубера
It is found along the Pacific coast from the Ecuadorian province of Manabí south to the Peruvian region of La Libertad. It inhabits coastal deserts and dry tropical forests, at altitudes up to 300 m.

(Epictia teaguei)
Northern Blind Snake
Стрункий сліпун північний
It is found in northern Peru. It inhabits humid mountain rainforests at altitudes up to 2,350 m.

(Epictia tenella)
Guyana Blind Snake
Стрункий сліпун гвіанський
It is found on Trinidad in the Caribbean, and in South America, where it ranges from Guyana south to Brazil and north-western Peru. It inhabits humid lowland tropical forests, on plantations, in gardens, and near human settlements.

(Epictia tesselata)
Tschudi's Blind Snake
Стрункий сліпун Чуді
It is found on the Pacific coast of Peru, in the vicinity of Lima. It inhabits the coastal Sechura Desert and dry scrublands and is also found in parks, gardens, and on sandy beaches.

(Epictia tricolor)
Three-colored Blind Snake
Стрункий сліпун триколірний
It is found on the western and eastern slopes of the highlands of the Peruvian Andes, from Cajamarca to Ancash. It inhabits high-altitude meadows at elevations of 2,000–3,300 m.

(Epictia vellardi)
Pantanal Threadsnake
Стрункий сліпун Велларда
It is found in Argentina (Formosa and Chaco provinces), Brazil, and Paraguay.

(Epictia venegasi)
It is known from the type locality in the vicinity of Cachachi-Moyan in the Cajabamba Province of northern Peru, at an elevation of 2,551 m.

(Epictia vindumi)
Vindum's Worm Snake
Стрункий сліпун Віндума
It is endemic just to the northern portion of Mexico’s Yucatan Peninsula, in the states of Yucatán and Quintana Roo, at elevations of up to about 30 m.

(Epictia vonmayi)
It is known from the type locality in the Querocoto District of Chota Province in northern Peru, at an elevation of 2,069 m. It inhabits mountain bushes and fields.

(Epictia vanwallachi)
It is known from the type locality in Pataz Province of northern Peru, at an elevation of 1,292 m.
The genus (Epictia) also includes: (Epictia wynni), (Epictia unicolor), Eleven-striped Blind Snake (Epictia undecimstriata), South American Blind Snake (Epictia signata), Red-lined Blind Snake (Epictia rubrolineata), (Epictia rioignis), Peru Blind Snake (Epictia peruviana), Dark Blind Snake (Epictia melanura), (Epictia martinezi), (Epictia hobartsmithi), Guayaquila Blind Snake (Epictia guayaquilensis).

(Habrophallos collaris)
Collared Blind Snake
Стрункий сліпун ошийниковий
It is found in French Guiana and Suriname. It inhabits primary tropical forests at elevations of 0–450 m.

(Mitophis calypso)
Samana Threadsnake
Стрункий сліпун саманський
It is endemic to the Samaná Peninsula in the Dominican Republic on the island of Hispaniola. It inhabits tropical forests, on plantations and in gardens and meadows.
The genus (Mitophis) also includes: Thomas’ Blind Snake (Mitophis pyrites), Haitian Border Threadsnake (Mitophis leptepileptus), Martin Garcia Threadsnake (Mitophis asbolepis).

(Rena boettgeri)
Cerralvo Island Threadsnake
Стрункий сліпун Беттгера
It is found in the Cape Region of Baja California Sur and also on Isla Cerralvo in the Municipality of La Paz.

(Rena dulcis)
Texas Blind Snake
Стрункий сліпун техаський
It is endemic to the south-western United States and adjacent northern Mexico.

(Rena humilis)
Western Threadsnake
Стрункий сліпун західний
It is native to the southwestern United States and northern Mexico. It is found in deserts and scrub where the soil is loose enough for burrowing.

(Rena segrega)
Trans-Pecos Blindsnake
Стрункий сліпун транс-пекоський
It is found in the United States (southeastern Arizona, Texas, and New Mexico) and in Mexico (San Luis Potosí, northern Coahuila, Nuevo León, Durango, and Chihuahua).

(Rena unguirostris)
Southern Blind Snake
Стрункий сліпун південний
It is found in Argentina, Bolivia, and Paraguay.

(Rena maxima)
Giant Blind Snake
Стрункий сліпун гігантський
It is found in the Mexican states of Guerrero, Morelos, Oaxaca, and Puebla.
The genus (Rena) also includes: (Rena klauberi), Iverson’s Blindsnake (Rena iversoni), Duges’ Threadsnake (Rena dugesii), Michoacán Slender Blind Snake (Rena bressoni).

(Siagonodon borrichianus)
Degerbol's Blind Snake
Стрункий сліпун Дегербьоля
It is found in western Argentina, where it inhabits shrubland.

(Siagonodon septemstriatus)
Seven-striped Blind Snake
Стрункий сліпун семисмугастий
It is found in Bolivia, northern Brazil (Amazonas, Pará, Roraima), French Guiana, Guyana, Suriname, and south-eastern Venezuela. It inhabits forest at elevations of 0–500 m.

(Siagonodon cupinensis)
Mato Grosso Blind Snake
It is found in the Brazilian states of Amapá, Mato Grosso, and Pará, and in Suriname, where it inhabits savanna habitats.
The genus (Siagonodon) also includes: (Siagonodon exiguum).

(Tetracheilostoma bilineatum)
Two-lined Blind Snake
Стрункий сліпун дволінійний
It is found on Martinique, Saint Lucia, and Barbados in the Lesser Antilles.

(Santa Lucía Threadsnake)
Santa Lucía Threadsnake
Стрункий сліпун сент-люсійський
It is endemic to the Caribbean island of Saint Lucia and inhabits forest at elevations of 40–60 m.

(Tetracheilostoma carlae)
Barbados Threadsnake
Стрункий сліпун барбадоський
It is endemic to the island of Barbados in the Caribbean Sea.

(Tricheilostoma bicolor)
Two-colored Blind Snake
Стрункий сліпун двоколірний
It is found in Mali, Guinea, Ivory Coast, Burkina Faso, Ghana, Togo, Benin, Nigeria, Niger, and Chad, and possibly also in Cameroon. It inhabits wet and dry savannas at elevations of up to 700 m.
(Tricheilostoma sundewalli)
Sundevalls Worm Snake
Стрункий сліпун Сундеваля
It is found in Cameroon, Central African Republic, Ghana, Nigeria, and Togo.
The genus (Tricheilostoma) also includes: (Tricheilostoma kongoensis), (Tricheilostoma greenwelli), Sudan Blind Snake (Tricheilostoma dissimilis), (Tricheilostoma broadleyi).
(Rhinoleptus koniagui)
Villiers' Blind Snake
Стрункий сліпун Вільє
It is found in West Africa in Senegal (Bonghari and Casamance) and Guinea (Kouroussa and Youkounkoun). It inhabits dry savannas and gallery forests at elevations of 0–450 m.
(Rhinoguinea magna)
It is endemic to southern Mali.

(Trilepida anthracina)
Bailey's Blind Snake
Стрункий сліпун Бейлі
It is found in Ecuador at Abituagua, Balzapamba, Baños de Agua Santa, and Zamora. It inhabits forest at elevations of 1,000–1,800 m.

(Trilepida brasiliensis)
Brazilian Blind Snake
Стрункий сліпун бразильський
It is found in the Brazilian states of Bahia, Ceará, Goiás, Maranhão, Mato Grosso, Mato Grosso do Sul, Minas Gerais, Paraíba, Piauí, Rondônia, and Tocantins, where it inhabits cerrado savannas and dry caatinga thickets.

(Trilepida acutirostris)
It is known from the state of Tocantins, Brazil, where it lives in cerrado savannas.

(Trilepida brevissima)
Caqueta Blind Snake
It is known from two localities in the Colombian Andes.

(Trilepida dimidiata)
Dainty Blind Snake
Стрункий сліпун витончений
It is found in Guyana, Suriname, and the Brazilian state of Roraima, and possibly also in south-eastern Venezuela. It inhabits humid tropical forests and savannas at elevations of 20–300 m.

(Trilepida dugandi)
Dugand's Blind Snake
Стрункий сліпун Дуганда
It is found in the Atlántico Department of Colombia. It inhabits forest and shrubland at elevations of up to 800 m, but has also been found in rural gardens.

(Trilepida fuliginosa)
It is found in the Brazilian states of Bahia, Minas Gerais, and Tocantins, where it inhabits cerrado savannas.

(Trilepida jani)
It is endemic to Brazil and is found in the Serra do Espinhaço Mountains in the state of Minas Gerais, at elevations of up to 1,700 m. It inhabits high-altitude meadows.

(Trilepida joshuai)
Joshua's Blind Snake
Сліпун Джошуа
It is found in the Colombian departments of Antioquia, Caldas, and Valle del Cauca. It inhabits forest at elevations of 1,600–2,200 m.

(Trilepida koppesi)
Amaral's Blind Snake
Сліпун Амарала
It is endemic to Brazil, where it is found in the Distrito Federal and the Brazilian states of Bahia, Goiás, and Mato Grosso. It inhabits grassland and savanna.

(Trilepida macrolepis)
Big-scaled Blind Snake
Великолускатий стрункий сліпун
It is known only from Santander Department, Colombia. It inhabits humid mountain rainforests at elevations of 1,750–1,824 m.

(Trilepida salgueiroi)
Espíritu Santo Blind Snake
It is found in the Brazilian states of Bahia, Espírito Santo, Minas Gerais, and Rio de Janeiro. It inhabits Atlantic forests and caatinga thickets.

(Trilepida pastusa)
Pastuso Thread Snake
It is endemic to Ecuador.
The genus (Trilepida) also includes: Santander Blind Snake (Trilepida nicefori), Venezuela Blind Snake (Trilepida affinis).

(Namibiana gracilior)
Slender Thread Snake
It is found in southern Namibia and western South Africa. It inhabits thickets of bushes.

(Namibiana occidentalis)
Western Thread Snake
Західний стрункий сліпун
It is found in Namibia and north-western South Africa. It inhabits the Namib Desert, semi-deserts, and dry savannas at elevations of 50–1,500 m.

(Namibiana labialis)
Damara Thread Snake
Стрункий сліпун дамарський
It is found in north-western Namibia and southern Angola. It inhabits dry savannas at elevations of 300–1,200 m.

(Namibiana rostrata)
Angolan Beaked Thread Snake
Дзьобатий сліпун ангольський
It is found in southern Angola, inhabiting dry savannas with spurges and baobabs, as well as wooded miombo savannas. It occurs at elevations of up to 1,300 m.

(Namibiana latifrons)
Benguela Worm Snake
It is endemic to the southwestern coast of Angola. It inhabits coastal sand dunes covered with shrub vegetation dominated by spurges.

(Myriopholis algeriensis)
Gambia Blind Snake
Стрункий сліпун гамбійський
It is known from a number of localities in Gambia, southern Morocco, Algeria, southern Tunisia, Mauritania, northern Mali, and north-eastern Niger. It inhabits the Sahara Desert, where it is found in oases and wadis at elevations of up to 1,100 m.

(Myriopholis blanfordi)
Sindh Thread Snake
Стрункий сліпун Бланфорда
It is found in India, Pakistan, Afghanistan, southern Iran, and possibly the Arabian Peninsula.

(Myriopholis burii)
Arabian Blind Snake
Стрункий сліпун аравійський
It is found in southwestern Saudi Arabia and southwestern Yemen at elevations of 1,350–1,460 m.

(Myriopholis cairi)
Cairo Blind Snake
Стрункий сліпун каїрський
It is found in Egypt, Eritrea, Ethiopia, Mauritania, Niger, Somalia, Sudan, Uganda, and possibly in Libya. It inhabits dry shrubbery, as well as fields and gardens near water bodies, at elevations of up to 400 m.

(Myriopholis ionidesi)
Ionides’ Worm Snake
Стрункий сліпун Іонідеса
It is known from a number of localities in south-eastern Tanzania and northern Malawi and Mozambique. It inhabits miombo woodlands at elevations of up to 130 m.

(Myriopholis longicauda)
Long-tailed Thread Snake
Стрункий сліпун довгохвостий
It is found in eastern Botswana, Kenya, Mozambique, southern Somalia, north-eastern South Africa, Tanzania, Zambia, and Zimbabwe. It inhabits savannas.

(Myriopholis macrorhyncha)
Hook-snouted Worm Snake
Стрункий сліпун гачконосий
It is found in isolated populations across northern Africa and in south-western Asia. It inhabits Mediterranean thickets, semi-deserts, and dry savannas, and is found near human settlements at elevations of up to 1,250 m.

(Myriopholis macrura)
Boulenger's Blind Snake
Стрункий сліпун Буленжера
It is endemic to the island of Socotra. It inhabits a variety of habitats, including palm groves, open sandy areas, rocky areas, shrub thickets, rocky hills with little vegetation, and wadis.

(Myriopholis narirostris)
Sahel Thread Snake
Стрункий сліпун сахельський
It is found in West Africa (Ghana, Togo, Nigeria, and possibly Guinea) and in Middle Africa (Central African Republic). It inhabits wet savanna and dry forest.

(Myriopholis nursii)
Nurse's Blind Snake
Стрункий сліпун Нерса
It is endemic to the southern Arabian Peninsula and the Horn of Africa. It inhabits savannas and agricultural lands with moist soil at elevations of 50–1,525 m.

(Myriopholis parkeri)
Parker’s Worm Snake
Стрункий сліпун Паркера
It is found in Ethiopia and Kenya. It inhabits dry meadows and dry acacia and commiphora thickets.

(Myriopholis rouxestevae)
Roux-Estève’s Worm Snake
It is found in Senegal, Guinea, and Uganda. It inhabits agricultural areas.

(Myriopholis wilsoni)
Wilson's Blind Snake
Стрункий сліпун Вілсона
It is endemic to the island of Socotra in Yemen. It inhabits forest and shrubland at elevations from sea level to 995 m.
(Myriopholis)
It is a genus of snakes in the family Leptotyphlopidae. Representatives of this genus occur in Africa, West Asia, and South Asia.
Yemen Blind Snake (Myriopholis yemenica), Tana Worm Snake (Myriopholis tanae), (Myriopholis perreti), (Myriopholis occipitalis), (Myriopholis mackayi), Lanza’s Worm Snake (Myriopholis lanzai), Socotra Island Blind Snake (Myriopholis filiformis), Eritrean Worm Snake (Myriopholis erythraeus), Scortecci’s Blind Snake (Myriopholis braccianii), Bouet’s Worm Snake (Myriopholis boueti), White-bellied Worm Snake (Myriopholis albiventer), Adler’s Worm Snake (Myriopholis adleri).

(Leptotyphlops conjunctus)
Cape Thread Snake
Стрункий сліпун капський
It is found in central areas of the Eastern Cape Province of South Africa and elsewhere in north-eastern and eastern South Africa, as well as in Lesotho and Eswatini. It inhabits meadows at elevations of up to 1,600 m.

(Leptotyphlops distanti)
Distant's Thread Snake
Стрункий сліпун Дістанта
It is found in Eswatini, southern Mozambique, and South Africa. It inhabits savanna and shrubland at elevations of 250–1,600 m

(Leptotyphlops emini)
Emin Pasha’s Worm Snake
Стрункий сліпун Еміна
It is found in the Democratic Republic of the Congo, Kenya, Sudan, Tanzania, Uganda, and northern Zambia. It inhabits savanna and shrubland at elevations of 650–1,370 m.

(Leptotyphlops incognitus)
Incognito Thread Snake
The species occurs in southern Zambia and Malawi, northern and eastern Zimbabwe, central and southern Mozambique, north-eastern South Africa, and Eswatini, and possibly also eastern Botswana. It inhabits dry tropical forests, wet savannas and grasslands, under stones and fallen leaves, and in termite mounds.

(Leptotyphlops jacobseni)
Jacobsen's Thread Snake
Стрункий сліпун Якобсена
It is endemic to South Africa, occurring in southern Limpopo and northern Mpumalanga. It inhabits high-altitude meadows, under rocks, and old termite mounds at elevations of 1,300–1,700 m.

(Leptotyphlops kafubi)
Shaba Thread Snake
It occurs in southern and central Africa, with confirmed records from Angola, Democratic Republic of the Congo, and Zambia. Its preferred habitat is savannas, but it may also be found in disturbed or artificial terrestrial environments.

(Leptotyphlops macrops)
Goggle-eyed Worm Snake
It is found exclusively along the coast of southern Kenya and northern Tanzania in East Africa. It inhabits coastal forests and humid savannas at altitudes of up to 100 m.

(Leptotyphlops merkeri)
Merker's Thread Snake
Стрункий сліпун Меркера
It is found on the mountain plateaus of the East African Plateau in Kenya and Tanzania. It inhabits wet and dry savannas, grasslands, and shrublands at altitudes of up to 1,500 m.

(Leptotyphlops nigricans)
Black Thread Snake
Чорний стрункий сліпун
It is endemic to the Western and Eastern Cape Provinces of South Africa. It inhabits bushes, meadows, and savannas.

(Leptotyphlops scutifrons)
Peter's Thread Snake
Стрункий сліпун Пітерса
It is found in Angola, Namibia, Botswana, Zimbabwe, South Africa, Eswatini, and western Lesotho. It inhabits wet and dry savannas, grasslands, and coastal scrub.

(Leptotyphlops sylvicolus)
Forest Thread Snake
Стрункий сліпун лісовий
It is endemic to South Africa, occurring in the provinces of KwaZulu-Natal and the Eastern Cape. It inhabits exclusively wooded areas.

(Leptotyphlops telloi)
Tello's Thread Snake
Стрункий сліпун Телло
It is found in the Lubombo Mountains on the border of Eswatini and Mozambique, and probably also in South Africa. It inhabits montane savannas at altitudes of 125–700 m.
(Leptotyphlops)
This is a genus of nonvenomous blind snakes, endemic to Africa and found throughout the continent.
Pungwe Thread Snake (Leptotyphlops pungwensis), Pitman’s Thread Snake (Leptotyphlops pitmani), Pemba Worm Snake (Leptotyphlops pembae), Black-tip Worm Snake (Leptotyphlops nigroterminus), Mbanja Worm Snake (Leptotyphlops mbanjensis), Uvira Worm Snake (Leptotyphlops latirostris), Mount Kenya Worm Snake (Leptotyphlops keniensis), Howell’s Worm Snake (Leptotyphlops howelli), Emin Pasha’s Worm Snake (Leptotyphlops emini), Ethiopian Worm Snake (Leptotyphlops aethiopicus).
(Epacrophis drewesi)
Drewes’ Worm Snake
Сліпун Древеса
It is endemic to Kenya. It inhabits the transition zone between East African evergreen shrublands and Acacia–Commiphora bushland at an altitude of 1,300 m.
The genus (Epacrophis) also includes: Lamu Worm Snake (Epacrophis boulengeri), Reticulate Blind Snake (Epacrophis reticulatus).